What Is the Hybridization of the Central Atom in Sicl4
SiCl4 So3 TeCl2 HCN. What is the hybridization about the central atom in nocl.
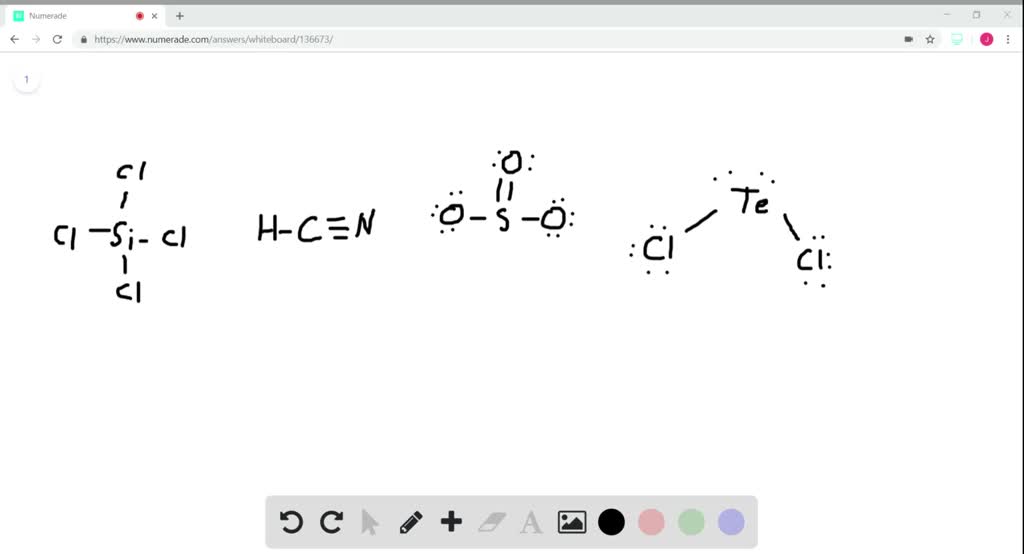
Solved What Is The Hybridization Of The Central Atom In A Mathrm Sicl 4 Mathbf B Mathrm Hcn Mathbf C Mathrm So 3 Mathbf D Mathrm Tecl 2
The bond angle of SiCl4 is 1095º.

. A single bond double bond triple bond or free pair are all one group. Draw them yourself and count the total number of groups including paired and unpaired electrons around the central atom. So the hybridization of Si in SiCl4 is sp3.
A three-step approach for drawing the SiCl4 molecular can be used. Hybridization What are the approximate bond angles in this substance. The central atom Si is attached to four Cl atoms through four sigma bonds.
The correct order of hybridization of the central atom in the following species is. Please explain 1 See answer Advertisement Advertisement klettkarrie is waiting for your help. So the steric number of Si atom is 4.
A SiCl4 b HCN c SO3 d TeCl2. What is the hybridization of the central atom in aSiCl4 b HCN cSO3 dTeCl2. Shown here are three pairs of hybrid orbitals with each set at For each pair determine the type of hybridization if any that could lead to hybrid orbitals at the specified angle.
What is the hybridization of the central atom in. What is the hybridization of sicl4. The hybridization of the SiCl4 molecule is Sp 3.
The number of groups is matched with the hybridization below. What is the hybridization of the central atom in the following molecules. SiCl4 is tetrahedral and the central Si atom is sp3 hybridised.
SiCl4 So3 TeCl2 HCN. The overall formal charge in Silicon tetrachloride is zero. SiCl4 is nonpolar in nature although its bonds are polar.
There is no lone electron pair on Si atom. Sp sp2 or sp3. What is the hybridization of the central atom in each of the following.
What is the hybridization of the central atom in a SiCl4b HCN c SO3 d TeCl2. N H 3 P t C l 4 2 P C l 5 a n d B C l 3 is. The hybridization of the central atom in NOCl is sp2.
Hence to form 4 sigma bonds four hybridized orbitals are required. There is no lone electron pair on Si atom. Add your answer and earn points.
What is the hybridization of the central atom in BeCl2. Key Points To Consider When drawing The SiCl4 Molecular Geometry. Up to 256 cash back What is the hybridization of the central atom in a SiCl 4 b HCN c SO 3 d TeCl 2.
A 305 ms b 075 ms c 2132 ms d 197 ms. 2 groups -- sp. Im not gonna draw the Lewis structures but Ill give you a key.
What is the hybridization of the central atom in each of the following. Experts are tested by Chegg as specialists in their subject area. Hybridisation is an important concept that can be used very effectively to explain the shape of question_answer.
The central atom Si is attached to four Cl atoms through four sigma bonds. What is the hybridization of the central atom in SiCl4. The second step is to calculate the SiCl4 hybridization and the third step is to give perfect notation.
So the hybridization of Si in SiCl4 is sp3. So the steric number of Si atom is 4. Bond angles B.
We review their content and use your. Hence to form 4 sigma bonds four hybridized orbitals are required. SiCl4 So3 TeCl2 HCN.
The total valence electron is available for the Silicon tetrachloride SiCl4 lewis structure is 32. Math Chemistry Biology Programming Arts History BusinessLanguage Spanish EnglishTipsReviewBlog Home What the hybridization the central atom each the following December 26 2021 thanh What the hybridization the central. Find the velocity of a helium atom at the same temperature.
Who are the experts. Indicate the hybridization of the central atom in a BCl3 b AlCl4- c CS2 d GeH4. What is the hybridization of the central atom in each of the following.
The first step is to sketch the molecular geometry of the SiCl4 molecule to calculate the lone pairs of the electron in the central silicon atom. SiCl4 is tetrahedral and the central Si atom is sp3 hybridised. Chemistry questions and answers.
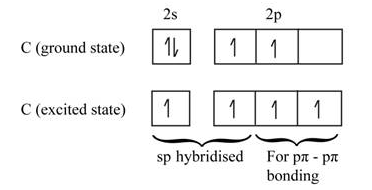
Answered What Is The Hybridization Of The Bartleby
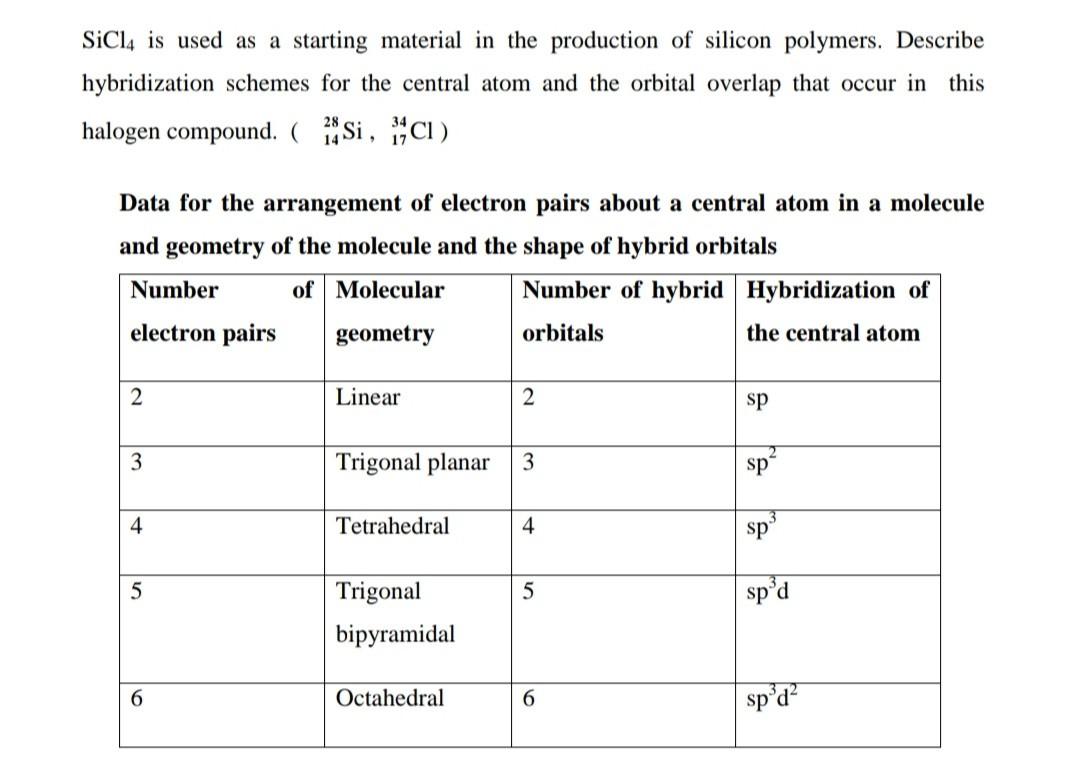
Solved Sicl4 Is Used As A Starting Material In The Chegg Com

Sicl4 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
What Is The Hybridization Of The Central Atom In Sicl4 Quora
No comments for "What Is the Hybridization of the Central Atom in Sicl4"
Post a Comment